Polyamide (PA) 6 is widely used in several industries and therefore economically very important. In its current industrial production process, first KA oil (a mixture of cyclohexanol and cyclohexanone) is obtained from cyclohexane ineffective oxidation (ca. 4% cyclohexane conversion to assure a reasonable selectivity, ca. 85%), and then converted, at high pressure and 150 ºC and in the presence of copper and chromium oxides, to cyclohexanone which is then used to form caprolactam, the monomer for produce PA 6.
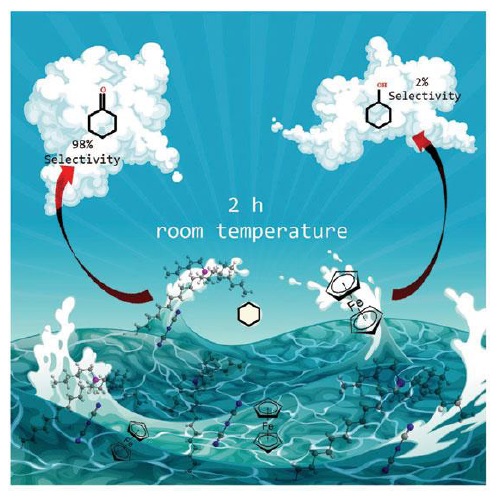
Luísa Martins and Ana Ribeiro, Instituto Superior de Engenharia de Lisboa and Instituto Superior Técnico, Lisbon, Portugal, have developed a method for the oxidation of cyclohexane with tert-butyl hydroperoxide (70% aqueous solution) in the eco-friendly phosphonium ionic liquid medium, [P6.6.6.14][DCA], and in the presence of catalytic amounts of ferrocene at room temperature. This reaction is rather selective (> 98%), yielding only cyclohexanone with total TOFs up to 10 000 h-1 and does not require the use of VOC solvents, additives or heating.
This is the first time that a phosphonium ionic liquid has been successfully used as a solvent for the oxidative functionalization of alkanes. In fact, the combination of a commercial iron-complex catalyst (ferrocene) and well-adjusted unconventional reaction conditions have led to the development of a highly selective, fast and reusable catalytic system for the mild oxidation of cyclohexane. Moreover, the found [Fe(C5H5)2]/ [P6.6.6.14][DCA] catalytic system can be of applied significance to produce polyamide 6.
For more information on this study, please visit: http://www.eurekaselect.com/151792