This article by Dr. Andrea Nicolini et al. is published in Current Medicinal Chemistry, Volume 25, 2018
Breast cancer, in the stage with distant metastases, is an incurable disease with a 5 years survival rate of about 5-10% (1). Immunotherapy has recently improved survival in metastatic breast cancer patients with breast cancer harbouring the expression of HER 2 receptor. Particularly in HER 2 positive patients pertuzumab plus trastuzumab and docetaxel is the recommended first line regimen and in triple negative breast cancer patients (TNBC) bevacizumab plus paclitaxel or docetaxel is a reasonable option. In the metastatic breast cancer patients responding to conventional antiestrogens without or with HER 2 receptor, the addition of interferon beta-interleukin 2 sequence importantly prolongs quality and length of survival in a pilot study of 42 patients. In fact, in all patients median progression-free survival (PFS) from the beginning of hormone-therapy was 33 months, (Fig.
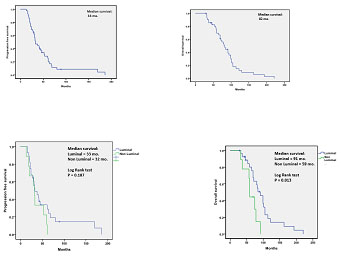
In the non-luminal sub-type, median PFS was 32 months and median OS was 59 months. Therefore, a relevant PFS and OS improvement occurred both in luminal and in non-luminal molecular subtypes compared with that expected in similar populations. In fact, equal or less than 10 months [
For more information, please visit: http://www.eurekaselect.com/159672